Introduction
Born with syndactyly, I have suspected my mother’s antibiotics exposure as the cause. Medication prescription to pregnant women should consider drug teratogenicity and maternal pharmacokinetics variations. This report will discuss the need to balance maternal advantages and foetal harms when managing medical conditions during pregnancy. Physicians need more situational-based guidance beyond the current drug classification system, and maternal communication and a comprehensive understanding of drug risks are crucial in consultations. Altering the drug dosages and schedules to keep up stable maternal condition is vital.
Classification of Drugs according to Foetal Risk
Teratogens are agents that induce or increase congenital malformations in functional or structural aspects. (Miller, Peters, and Schaefer, 2007). Many neonates have retarded growth or functional abnormalities from teratogenic effects (Gilbert-Barness, 2010), including intrauterine growth restriction and postnatal effects (Vickers and Brackley, 2002). As misunderstanding of teratogenic risk may cause unnecessary foetal morbidity and mortality, comprehensive drug utilisation guidelines are needed (Koren, Pastuszak, and Ito, 1998).
In 1979, the US Food and Drug Administration (FDA) classified drugs with ‘Use-in-Pregnancy Ratings’ (Table 1), based on the level of known teratogenicity. The Teratology Society suggested replacing it with statements involving developmental toxicity and potential teratogenic risks (Teratology Society Public Affairs, 1994). This system was criticised for being susceptible to misinterpretation and misapplication (Andrade et al., 2004). In June 2015, the Pregnancy and Lactation Labeling Rule (PLLR) replaced the original categorisation system. The pregnancy section in PLLR is classified further into ‘Risk Summary,’ ‘Clinical Consideration,’ and ‘Data’ (human and animal) (Bookstaver et al., 2015).
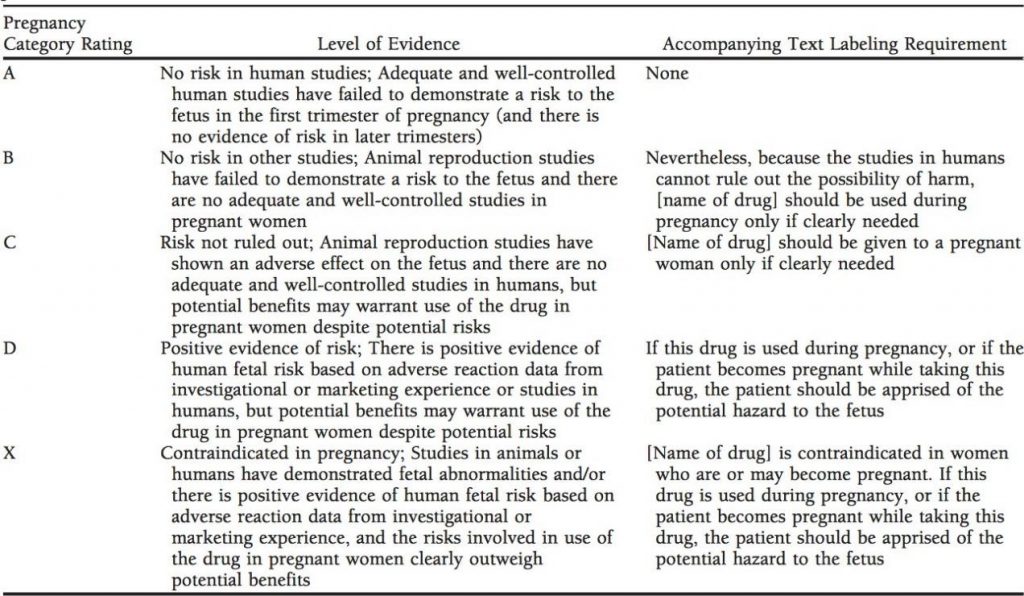
Table 1. FDA Pregnancy Category Ratings with Required Package Labeling Statements Prior to June 2015 (Bookstaver et al., 2015)
Drugs are placed into different categories with similar definitions in the FDA, Swedish, and Australian systems. The FDA system is more restrictive about drugs in category A and B described in Table 1, thus causing higher curettage rates (DemİR, Arici, DemİRal, and TunÇOk, 2012). Teratogenic drug safety is not effectively supported in all existing systems due to the lack of dose and pregnancy period in references (Dhombres, Fung, Rodriguez, and Bodenreider).
Effects of Pregnancy on Pharmacokinetics
Pharmacokinetics (PK) refers to the time course of drug absorption, distribution, metabolism, and excretion to characterise therapeutic dosage and adverse effects of drugs (Gibaldi, 1976). Physiological and hormonal changes begin mostly in the first trimester and increase linearly until parturition (Anger and Piquette-Miller, 2008). Variations in maternal physiology affect PK and efficacy of drugs like transplacental passage (Vickers and Brackley, 2002). With changes in body drug concentration, physicians should alter the drug schedules or dosages (Little, 1999).
Absorption
Elevated serum progesterone in the third trimester (Dawood, 1976) reduces small intestine motility and gastric emptying, resulting in a longer time for drugs to reach a high concentration in plasma. An increase in mucus production and gastric emptying time will reduce drug absorption, meaning that the efficacy of oral drugs will decrease. Nausea related to pregnancy is also a concern for drug absorption (Dawes and Chowienczyk, 2001).
Distribution
The total plasma volume increases with higher total body water content and the plasma protein albumin concentration falls during pregnancy. With fewer albumin binding sites, free active drug dose is raised and the volume of distribution of hydrophilic drugs is increased (Dawes and Chowienczyk, 2001). The dose of lipophilic drugs which cross the placenta rapidly will also increase as body fat increases in pregnancy (Anger and Piquette-Miller, 2008).
Metabolism
Hormone level variation in pregnancy down-regulates some enzymes. Extrahepatic enzymes like cholinesterase also diminish metabolic activity (Little, 1999). The greater hepatic blood flow serves as a coping mechanism to increase the enzyme metabolic capacity in pregnancy (Anger and Piquette-Miller, 2008). Hepatic cytochrome P-450 enzymes induced by progesterone or oestrogen also have a higher metabolic rate and are able to increase the rate of drug elimination (Dawes and Chowienczyk, 2001).
Excretion/ Elimination
During gestation, pulmonary function increases. This enhances drug eliminations through respiration. Renal excretion is still the most efficient elimination process (Reynolds and Knott, 1989). Higher renal blood flow increases glomerular filtration rate (Little, 1999), then increases renal elimination capacity and drug clearance rate (Bogaert and Thiery, 1983; Vickers and Brackley, 2002).
Teratogenic Drug Classes
Type of agent, frequency, dose, duration, and time of exposure is often proportional to teratogenic risks. The first half of pregnancy is the most vulnerable time for the growing foetus (Health, 2010).
Antibiotics
Antimicrobial agents can cross the placenta and cause toxicity. Aminoglycosides that inhibit protein synthesis show ototoxicity and nephrotoxicity in the newborn (Carter and Wilson, 1965). Aminoglycosides exposure during the first trimester reports irreversible bilateral congenital deafness. Long-term effects (e.g. asthma or eczema) in newborns are associated with antibiotic exposures during pregnancy. Permanent discolouration of teeth and bones are seen in tetracycline exposure beyond the second trimester (Bookstaver et al., 2015). Tetracyclines can cause palatine cleft, neural tube defects, and severe congenital cardiovascular abnormalities (Fiol, 2005).
Analgesic drugs
Nonsteroidal analgesics like aspirin that inhibits prostaglandin synthesis brings higher neonatal deformity rate and lower birth weight (Niederhoff and Zahradnik, 1983). Ibuprofen brings specific congenital anomalies like gastroschisis, cardiac septal defects, and orofacial clefts. Prolonged use leads to premature closure of foetal ductus arteriosus and cryptorchidism (Schaefer, Peters, and Miller, 2015).
Antidepressants
Antidepressants are avoided as cardiovascular effects and neural tube defects like exencephaly are seen (Vickers and Brackley, 2002). Infants with intrauterine exposure had higher birth weight and lower Apgar scores. Selective serotonin reuptake inhibitors exposure is consistent with impaired language function (Galbally, Watson, Boyce, Nguyen, and Lewis, 2020). Neonatal intoxication and postnatal withdrawal syndrome are teratogenic effects that can be seen in infants (Bethenod and Frederich, 1989). Extrapyramidal symptoms may also be seen in infants. (Levy and Wisniewski, 1974).
Benefits and Risks of Anti-epileptic Drugs (AEDs)
Antiepileptic drugs (AEDs) are involved with the production and detoxification rates of metabolites (Vickers and Brackley, 2002). Epilepsy treatment in pregnancy is challenging as foetal and maternal risk associated with maternal seizures must balance against AED’s potential teratogenic effects (Tomson, 2007).
Maternal benefits
Women with epilepsy (WWE) have higher seizure frequency during pregnancy, labour and delivery (Zahn, Morrell, Collins, Labiner, and Yerby, 1998). During pregnancy, seizures can cause maternal hypoxia and acidosis. Avoiding all seizure types in pregnancy is desirable for maternal physical wellbeing (Pennell, 2003).
It is unreasonable and unsafe for WWE to stop the medication due to their frequency and severity of epilepsy. Seizures during pregnancy have higher maternal risks, such as cardiovascular effects than drug exposure (Etemad, Moshiri, and Moallem, 2012). Loss of seizure control decreases plasma concentrations. There is a higher death rate among WWE due to seizures (Tomson, 2007).
WWE represent up to 0.5% of pregnant women, and 80% of these women prescribed with at least one AED are able to control their seizures. The frequency of seizures increases as the AED dose decreases to protect the foetus (Schaefer et al., 2015). Long-term AEDs administrations are able to control seizures (Dansky, 1991). Taking AEDs may cut seizures in the second or third trimester (Thomas, 2006).
Hepatic CYP inducers like carbamazepine inhibit sodium channels and block repetitive firing of action potentials. Reduction of AEDs can trigger status epilepticus, withdrawal responses and seizure clusters (French and Gazzola, 2013).
Foetal risk
The use of AED increases foetal major malformations risk two- to threefold (Schaefer et al., 2015). AED exposure in the last trimester brings the most harmful cognitive outcomes. Intrauterine growth retardation, microcephaly, infant motility, congenital malformations (neural tube defect, urogenital defect, and congenital heart disease), mild facial dysmorphism, inguinal hernia, limbs, hip, joint or renal anomalies are reported (Pennell, 2003). Postnatal abnormalities like visual disturbances and otitis media are observed. Developmental problems like autism spectrum disorders and verbal vs non-verbal abilities are also observed (Schaefer et al., 2015).
Foetal malformation rates increase with each AED prescribed. Polytherapy increases malformation risk. Some AEDs impair foetal folate absorption and cause postnatal developmental delay like lower intelligence quotient (IQ). Maternal use of hepatic-enzyme-inducing AED drugs may also induce foetal haemorrhagic disease (Zahn et al., 1998).
Prescription Issues for Medical Practitioner to Consider
Using medications during pregnancy poses a potential risk to the mother and foetus (Andrade et al., 2004). Sometimes physicians stop medications due to insufficient reliable information to avoid emergency that does more harm than the drug itself (Martin, 2017). However, the concern of underprescribing for pregnant women is higher than overprescribing. Not all drugs in pregnancy are useless or dangerous, with less than 1% being classified as teratogens (Haramburu, Miremont-Salamé, and Moore, 2000).
Physicians’ major concern is the foetal harm of agents (Anger and Piquette-Miller, 2008). Aware of pharmacokinetic alterations in pregnancy, clinicians should check evidence of drug choice, dosing, duration of therapy, and monitoring (Bookstaver et al., 2015). Careful weighing and discussion of benefits versus risks are required, especially for teratogens (Niederhoff and Zahradnik, 1983). Drugs could be prescribed in pregnancy when the benefits justify the risks (DemİR et al., 2012). In most situations, maternal benefits outweigh the child’s risks (Olesen et al., 1999).
The stable maternal disease is the most favourable outcome. Accurate patient information can avoid unnecessary anxiety and treatment non-compliance. Using the lowest effective dose of the safest drug for pregnant patients, polypharmacy avoidance, and health guidance like avoiding smoking and taking supplements are recommended. Counselling and detailed scanning can help to avoid unnecessary intervention (Vickers and Brackley, 2002).
Additionally, physicians should consider the consequences of ‘drug-free pregnancy’. For example, higher suicidal ideation and hospital admission rates have been reported in pregnant women who drop antidepressants abruptly. Abrupt discontinuation also causes re-emergence of primary psychiatric disorder and withdrawal symptoms such as perceptual disturbances in mainly vision and hearing. Medical practitioners should give effective counselling to reassure women who stop medicine due to their own fears or other’s advice to take therapy (Einarson, Selby, and Koren, 2001).
To promote safe and quality therapy, physicians should be familiarised with all possible teratogenic risks of drugs that treat the mother without affecting the foetus (Fiol, 2005). While teratogens taken at any developmental stage can potentially cause disruptions, most structural defects are seen in embryonic period exposure (Gilbert-Barness, 2010). Doctors may advise patients to avoid certain drugs in the first trimester. During the pre-embryonic period, drug exposure may cause pregnancy failure. Risk is minimal if pregnancy is viable. In the embryonic period, when organogenesis occurs, teratogenic effects like genital anomalies are observed. Serious functional but no structural abnormalities are seen in late pregnancy drug exposure (Vickers and Brackley, 2002). Increasing malformation rates with severity elevates the chance of spontaneous miscarriage or shortened life span (Chung, 2012).
Clinicians should individualise effective treatment as patients with the same dosage may translate into different clinical responses (Perucca, 2005). For example, genetic factors, like metabolism, can impact the manifestation of teratogenic effects (Schaefer et al., 2015).
Physicians should offer pregnant WWE with stable medication setting and follow-up sonography. They should also check liver and kidney function as well as haematological parameters, like plasma AED concentration of which protein binding changes in pregnancy (Schaefer et al., 2015). Suggest frequent nonstress tests for high-risk WWE (Seale, Morrell, Nelson, and Druzin, 1998). If an earlier child was born with anomalies and maternal drug exposure, clinicians should alter the regimen in the next pregnancy. A higher risk of recurrence is observed for drug-specific pharmacologic susceptibility (Schaefer et al., 2015).
Conclusion
Several categorisation systems are established for different drugs, including some teratogenic classes. As pharmacokinetics change with pregnancy and certain drug classes can have severe teratogenic effects, clinicians should prescribe medications only when maternal benefits outweigh the foetal risks.
Reflection
Not citing references manually like my assignment in Foundations, I used Endnote efficiently. I drew mind maps to outline searching keywords and structure instead of drafting directly from research papers. Establishing a well-planned timeline and word allocation, I finished my assignment earlier without a lot of irrelevant details.
At the beginning of my research, I misunderstood the tasks and tried to input drug classes into a categorisation system. I was so lost as the terms were unfamiliar and drugs in one class often overlapped with different categories. Through discussion with my colleagues, I realised my fault and made correction immediately.
Knowing only pharmacodynamics and pharmacokinetics as a beginner, I have gained a comprehensive understanding of drug safety compared to risks for several drug classes. I now understand that safe drug use is not impossible to achieve in pregnancy after reading significant powerful research.
As few of the resources in this report are not published recently, there may be existing confounding bias due to epigenetic changes. For prospective research, I will make sure to look for more modern papers in different databases other than PubMed.
Since animals are the testing subjects in some research, the difference in their biological mechanisms from humans may cause subject bias and affect external validity. I will mostly prefer studies that include human trials in future searches. I will also attempt to look for systemic reviews that incorporate varieties of cultural medications within international standards. With more practice, I will be confident in selecting research that achieved the CRAAP test standard.
Written by:
Weng Tong Wu AMSA Australia
